Products You May Like
Strip life down to its simplest components, you’re left with fragments of genes and proteins that rely on the cellular machinery of host organisms to reproduce – elements we call viruses.
As if to prove there is no honor among even the smallest of thieves, some virus-like entities known as mobile genetic elements (MGEs) can intrude on these cellular heists, taking advantage of the hijacking to further their own selfish interests (while occasionally bestowing a gift or two on their host).
Though it has long been known MGEs had to sidle up to their so-called ‘helper’ viruses while they invaded a host cell, nobody realized just how close some could get.
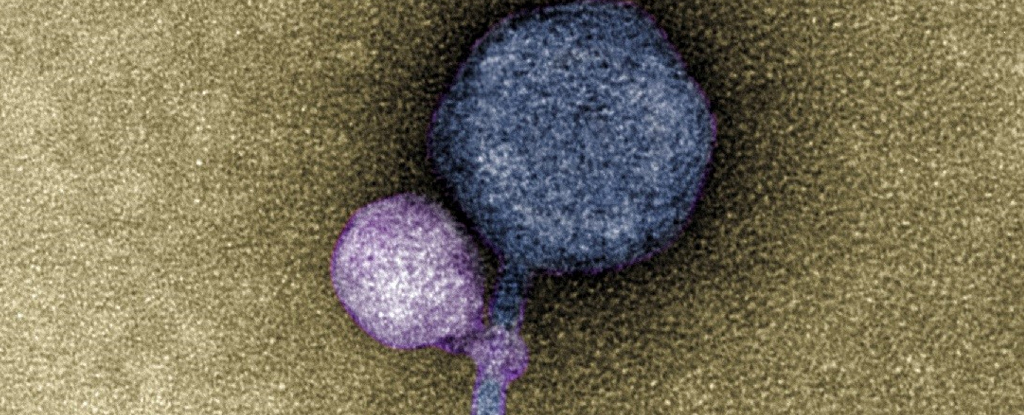
But now a study led by researchers from the University of Maryland Baltimore County (UMBC) has uncovered clear signs of MGEs latching onto the necks of their helpers as they infected a population of bacterial cells.
“When I saw it, I was like, ‘I can’t believe this,'” says biologist and lead author Tagide deCarvalho.
“No one has ever seen a bacteriophage – or any other virus – attach to another virus.”
On a fundamental level, all living things are essentially imperfectly-replicating sequences of nucleic acid. The fact our genes float inside microscopic garbage bags filled with a rich soup of proteins, fats, minerals, and carbohydrates is almost secondary – all of these additional materials ultimately ensure genes persist and replicate.
If a gene can manage this task without needing to encode its own complicated recipe of metabolic machinery, then kudos to them!
A kind of MGE known as satellite nucleic acid is as bare-bones as life gets. Lacking codes for producing critical elements such as a capsule, these sequences rely on the presence of other viruses to manufacture its machinery.
Enterobacteria phage P4 is one such example. Inside an Escherichia coli bacterium, P4 can either insert itself into the cell’s genome or simply hang out as a piece of extranuclear DNA known as a plasmid.
This all changes when it shares its host with Enterobacteria phage P2. Like a near-sighted nanny who just can’t say no, P2 knits protein overcoats on P4’s insistence, encapsulating all of the satellite nucleic acid fragments until the cell bursts and sends them off into the cold to infect new territory.
This latest discovery puts a new spin on the process. Investigating what they initially suspected to be mere contamination, the researchers uncovered a novel group of satellite phages attacking a species of Streptomyces bacterium.
Unlike Enterobacteria phage P4s, this phage can make its own coat. What it lacks is the pattern for ‘pants’ – the long tail-like appendage many phages use to poke their way through a host’s wall and membranes. For that, it needs its very own helper.
In studying electron microscope images of these unusual MGEs going about their business, deCarvalho and her team noticed the satellites weren’t just associating with a helper, but were ironically using their tails to grab ahold of the larger virus.
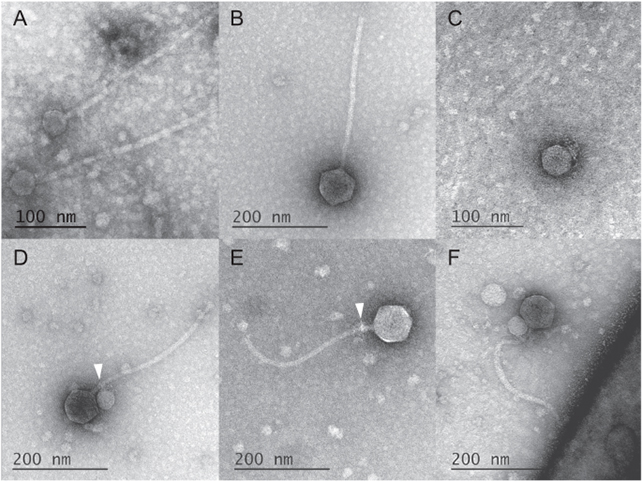
Some 40 out of the 50 helper phages the team counted had a satellite clinging to them like some kind of microscopic vampire. Among the 10 that happened to be satellite-free, frayed remnants of tell-tale fibers could still be seen wrapped around their throats (much like “bite marks”, the researchers say).
Further investigation revealed the satellite was missing another common viral tool. It had no way of weaving its genes into the host’s genome. Keeping in mind the MGE couldn’t preserve itself inside the host’s DNA, it makes sense to become a barnacle on a helper’s neck.
“Attaching now made total sense because otherwise, how are you going to guarantee that you are going to enter into the cell at the same time?” explains senior author Ivan Erill, a computational biologist at UMBC.
Given the finding was nearly dismissed as stray DNA polluting the experiment, it’s possible there are plenty of other examples of satellite-helper systems lurking in the seedy underbelly of the microscopic universe.
This research was published in The ISME Journal.