Products You May Like
Scientists have been able to observe a common interaction in quantum chemistry for the first time, by using a quantum computer to shadow the process at a speed 100 billion times slower than normal.
Known as a conical intersection, the interactions have long been known about, but are usually over in mere femtoseconds – quadrillionths of a second – making direct observations impossible to carry out.
A research team from the University of Sydney in Australia and the University of California, San Diego, instead monitored the reaction using a charged particle trapped in a field, allowing them to follow a version of the process that dragged on for a relative eternity.
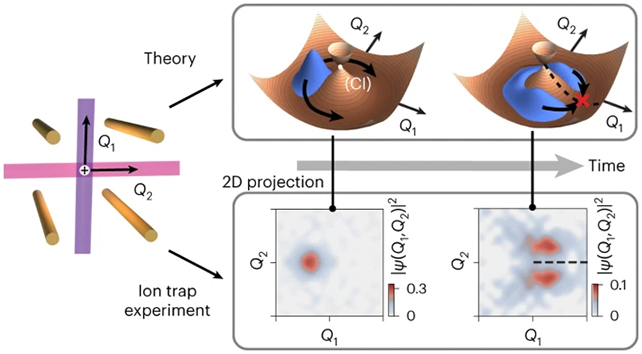
“Using our quantum computer, we built a system that allowed us to slow down the chemical dynamics from femtoseconds to milliseconds,” says Vanessa Olaya Agudelo, from the School of Chemistry at the University of Sydney.
“This allowed us to make meaningful observations and measurements. This has never been done before.”
Conical intersections describe the rapid transfer of energy between surfaces of potential energy inside molecules. As such, they are best described using the language and mathematics of quantum physics, involving overlapping fields and changing waves of particle behavior.
In chemical terms, the quantum reactions govern light-based reactions in all kinds of scenarios, such as photosynthesis and reactions in the human eye.
What made this current research possible was the special way the scientists were able to map the electron state change onto features of a system using a trapped ion quantum computer, where electric fields do the trapping and lasers do the manipulating.
Once this complex process had been carried out, the team was then able to slow everything down so it could be observed. The scientists compare this to running observations of aerodynamics on an airplane wing in a wind tunnel.
“Our experiment wasn’t a digital approximation of the process – this was a direct analogue observation of the quantum dynamics unfolding at a speed we could observe,” says Christophe Valahu, from the School of Physics at the University of Sydney.
As conical intersections are so common in photochemistry, the new research is going to be hugely useful in a lot of areas of research. It shows how new insights can be found through researchers from different fields of science working together.
More generally, quantum computers hold a lot of promise when it comes to simulating all kinds of reactions and interactions. A better understanding of the fastest and smallest events means we’ve got a better idea of how to make use of them.
“It is by understanding these basic processes inside and between molecules that we can open up a new world of possibilities in materials science, drug design, or solar energy harvesting,” says Olaya Agudelo.
“It could also help improve other processes that rely on molecules interacting with light, such as how smog is created or how the ozone layer is damaged.”
The research has been published in Nature Chemistry.
