Products You May Like
Long-lived fungi are the latest organisms to go under the microscope in search of new understandings as to why they don’t accrue life-limiting mutations, given their age.
Researchers at Wageningen University in the Netherlands set out to compare “the peculiarities” of multicellular growth in filamentous fungi. What they ended up with was a new hypothesis explaining how certain types of fungi keep a lid on freeloading mutations that accumulate in their thread-like mycelia; the root-like structures of fungal colonies.
The filaments of mushroom-forming fungi spend much of their long lives with two, separate nuclei, each containing one-half of a full set of chromosomes. Only in the gills of mushrooms moments before forming spores do the two haploid nuclei mesh together in a brief union to reproduce asexually.
Mutations in either nucleus rob the affected mycelium of its ability to fuse its spindly filament to another, forcing other mycelia to pay the cost in spore formation. Given enough time, mutated mycelia will dominate the fungus, reducing its ability to make spores at all.
First discovered in fast growing Neurospora crassa mold in 2016, the mutated nuclei were dubbed ‘cheaters’ for the way mutated mycelia can’t begin asexual fusion with their own filaments to form spores, but can piggyback off other fully functioning mycelia they may encounter.
This biological tension between individual cells and whole organisms echoes cancer in other organisms, where mutant cells hellbent on replicating grow so fast they harm the animal in which they arose, sometimes fatally.
“Because these [fungal] mutations are selected within the mycelium, but reduce the fitness of the mycelium as a whole, you can think of them as a kind of ‘nucleus cancers’,” explains evolutionary biologist and lead author Duur Aanen, of Wageningen University.
Aanen and colleagues compared fast-growing molds and long-lived mushroom mycelia that can live for hundreds of years. They suggest the latter use a special type of cell division called a ‘clamp connection’ to screen against selfish mutants, enabling them to live long lives without amassing too many genetic faults.
In this form of cell division, one of the filament’s haploid nuclei is interned in a holding bay until the cell can check its quality – and that filament fusion is possible.
“Both nuclei [are] continuously testing each other for the ability to fuse, a test that nuclei with mutations in fusion genes fail,” explains Aanen. “If the cell cannot fuse, it means a dead-end for the cell and thus the end of its nucleus.”
In humans and other animals, cancer develops after an organism accumulates enough genetic errors to unleash runaway cell division. Seeing those errors pile up over time, you’d think that creatures with long lifespans or large bodies would develop more tumors.
But elephants and whales defy that reasoning, deploying molecular tricks to repair damaged DNA, keep cell division in check, and suppress cancer.
These evolutionary solutions to ‘Peto’s paradox‘, as it’s known, are of great interest to scientists – or anyone who’d like to prevent cancer.
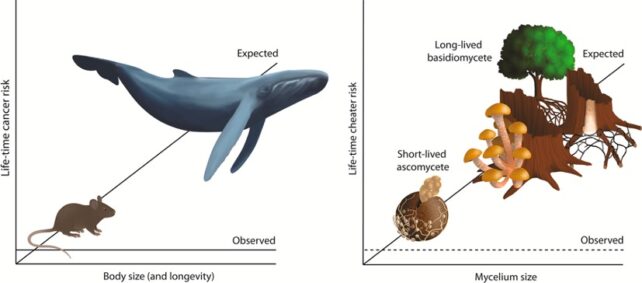
Though there are some parallels in this new study, fungi are strange beings, comprising a whole other kingdom of life to us animals. So there may be less chance of scientists finding some cellular machinery in fungi capable of quashing cancer that could be relevant to humans.
Yet we can still appreciate the many ways evolution has gifted organisms the tools to keep life chugging along, especially when it tries to thwart itself.
Check-mate, fungal cancer.
The study has been published Microbiology and Molecular Biology Review.