Products You May Like
Despite a global ban in place since 2010, atmospheric concentrations of five ozone-depleting chemicals have reached a record high.
Chlorofluorocarbons, or CFCs, are entirely man-made gasses used in a variety of applications, including refrigeration, air conditioning, or as chemical solvents. They have been increasingly regulated by a series of international treaties since the 1980s.
The 1987 Montreal protocol, which has been unanimously ratified, restricted the release of CFCs to the atmosphere where they contribute to the destruction of the ozone layer: a region high up in the stratosphere which absorbs harmful ultraviolet (UV) radiation and protects life below.
The goal of the Montreal protocol was to induce a decline in the atmospheric CFC concentration through controlling, and increasingly restricting, the production of these chemicals.
This has worked well for many ozone-depleting substances, which is why the ozone layer is slowly recovering. And so the recent increase in atmospheric concentrations of five CFCs is quite surprising.
Our findings, while worrying, should be considered an early warning. The impact of all five CFCs on the recovery of the ozone layer is still small.
Nevertheless, we do not fully understand where they are coming from, so this could change in the future, and we should not ignore the cumulative effect of these emissions on human health and the environment.
The global picture
Our team has been analyzing air samples from all over the world, focusing on so-called “background” sites that are far away from the sources of these CFCs, or in fact any industrial emissions.
An example is the Cape Grim observatory on the remote west coast of Tasmania. This is the basis for our assessment of the threat these chemicals pose, as it reveals global trends in their atmospheric concentration.
Our main findings for the period 2010-2020 were twofold.
First, concentrations of CFC-13 and CFC-113a continued their previously observed – and puzzling – increase. Rising concentrations of CFC-113a even accelerated around 2016.
Second, concentrations of CFC-114a and CFC-115 were stable since the 2000s, while those of CFC-112a had even started to decrease. However, all of them began increasing around 2013-2014.
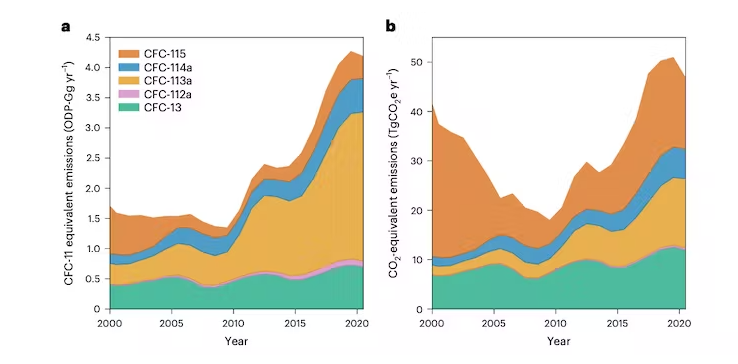
These observations, combined with additional knowledge about atmospheric circulation and how CFCs are removed from the atmosphere through chemical reactions, allowed us to estimate the global emissions of these five gasses.
Their damage to the ozone layer can be expressed through their ozone depletion potential, which states how much ozone would be destroyed compared to the same quantity of CFC-11, which is different for each CFC.
The result is a relief. Emissions between 2010 and 2020 only resulted in a very small loss of around 0.002 percent of global stratospheric ozone.
There is no time to relax, though, for two reasons.
All five CFCs are also potent greenhouse gasses and, once emitted, will remain in the atmosphere for decades to centuries. Their warming effect in 2020 was already approximately that of Switzerland’s total CO₂ emissions. And if those emissions continue on their upwards trajectory, their contribution to climate change will expand too.
The persistence of these gasses in the atmosphere must be taken seriously: all emissions are a legacy for future generations to contend with.
Tracking down the sources
The first step towards avoiding future emissions is to find out where the current ones are coming from. There were already some hints in previous studies, which we gathered and combined with our own information, such as on the exact timing of when emissions started accelerating.
We found that three of the five CFCs (CFC-113a, CFC-114a, and CFC-115) can be produced during the manufacture of other chemicals, which is allowed under the Montreal protocol, most notably hydrofluorocarbons or HFCs. HFCs have replaced CFCs for many applications as an ozone-friendly alternative.
However, like CFCs, they are greenhouse gasses and their production is now being reduced in many countries under the 2016 Kigali Amendment to the Montreal Protocol, which could reduce climate-related warming by 0.5 °C.
It’s likely that the CFCs are leaking out during the production process, where they are either used as a feedstock (a chemical ingredient to make another chemical) or as a result of incomplete conversion of the feedstock to the target chemical.
The production of HFCs really took off in developing countries after CFCs were banned in 2010, which is around the same time as the increase in emissions of these five CFCs.
The production of HFCs is predicted to further increase over the next few years, which could result in increasing emissions of these CFCs. CFC-113a is used to make at least one hydrofluoroolefin or HFO, which are alternatives to HFCs that don’t heat the climate and may be used long into the future.
Despite HFCs and HFOs being more benign alternatives to CFCs, there may still be some cost to ozone during their production if CFCs continue to leak into the atmosphere.
We were unable to find a plausible source for the other two CFCs, CFC-13 and CFC-112a. The fact that their emissions are increasing and we don’t know why is a concern in itself.
Time to revisit Montreal?
The Montreal protocol has been a huge success in mitigating emissions of ozone-depleting substances. Total CFC emissions are now only around 5 percent of their peak in the late 1980s.
Yet an increase in the atmospheric abundance of some CFCs is still at odds with the treaty’s goals – and their elimination, by clogging leaks in industrial processes, could present an easy win to reduce these country-sized emissions of ozone-depleting and climate-warming gasses.
It will take careful consideration by countries signed up to the protocol to find the necessary controls for quashing these trend-bucking emissions.
In the meantime, we will continue to use our eyes in the sky to monitor the progress of a whole host of Earth-damaging gasses.
Luke Western, Research Associate in Atmospheric Science, University of Bristol and Johannes Laube, Honorary Lecturer, Centre for Ocean and Atmospheric Sciences, University of East Anglia
This article is republished from The Conversation under a Creative Commons license. Read the original article.
