Products You May Like
A well-known bacterium could transform carbon dioxide from the air into a useful bioplastic, tackling two global problems in one swift move, using a prototype system designed by a team of chemical engineers in Korea.
Plastic-munching bacteria capable of breaking down plastic waste in a matter of hours have attracted much attention lately as a microscopic solution to the world’s growing plastic problem.
While cleaning up the mess we’ve already made is a huge priority, looking for new ways to make plastics from sources other than crude oil and its derivatives is also vital to reducing our dependency on fossil fuels.
Plastic polymers are long chains of repeating subunits strung together, and the backbone of these chains is most often carbon atoms.
Many chemical engineers have clued on to the bright idea that the rising levels of carbon dioxide in Earth’s atmosphere could be an untapped resource for making plastics or other carbon-based products, such as jet fuel or concrete – if only we could capture CO2 from the air and make something of it.
One way to convert CO2 gas into other useful carbon-containing compounds is with an injection of electricity in a reaction called electrolysis. But this method, while promising, mostly produces short-chain, starter compounds of only one to three carbon atoms. Making chemicals with longer carbon chains from CO2 is a harder and more inefficient task.
In this new effort, a team of chemical engineers at the Korea Advanced Institute of Science and Technology (KAIST) has developed a two-part system for transforming CO2 into a common type of bioplastic with the help of a bacterial species called Cupriavidus necator.
The first step of the system is an electrolyzer that converts gaseous CO2 into formate. Then, this is fed into a fermentation tank, where the bacteria get to work.
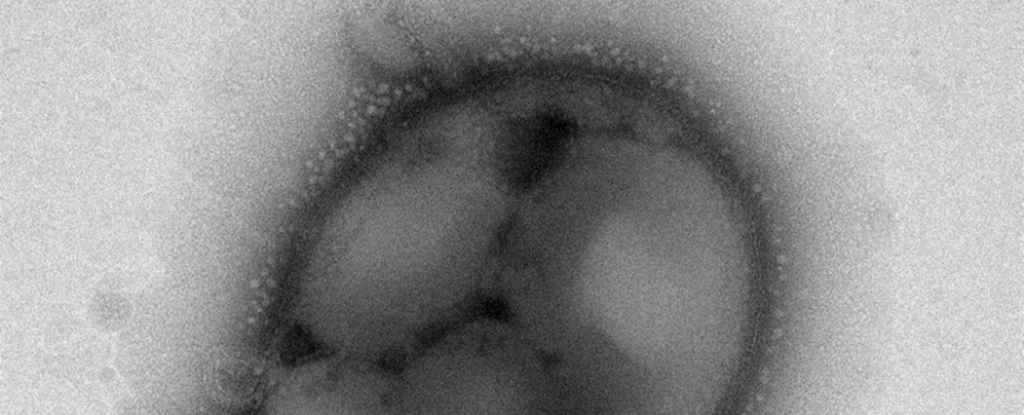
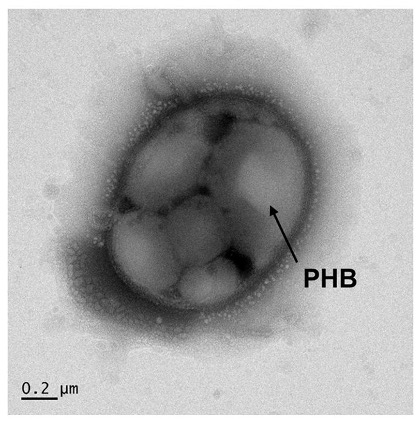
C. necator is well-known for its ability to synthesize carbon compounds such as poly-3-hydroxybutyrate or PHB, a type of biodegradable and compostable polyester, from other carbon sources.
In this case, C. necator gobbles up the formate feedstock from the electrolysis reaction and stockpiles granules of PHB – which can then be extracted from harvested cells.
The same solution circulates between the electrolysis reaction and the fermentation tank, with a membrane separating the two chambers so that the bacteria are isolated from the by-products of the electrolysis reaction.
If the system is powered by renewable energy, then it could be a fossil-fuel-free way of generating bioplastics that simultaneously makes use of CO2 – which fast needs to be scrubbed from the air to limit global heating.
Hyunjoo Lee and Sang Yup Lee, two biomolecular engineers at KAIST who led the study, are optimistic that their approach is scalable and could go some way to helping transform the way plastics get made.
“The results of this research are technologies that can be applied to the production of various chemical substances as well as bioplastics and are expected to be used as key parts needed in achieving carbon neutrality in the future,” they say.
While that remains to be seen, it seems like an option worth pursuing.
Lab experiments showed that C. necator cells in the hybrid system could synthesize so much PHB that after 120 hours or 5 days of operation, the polyester product represented up to 83 percent of the bacteria’s dry cell weight.
Based on these results, the researchers claim their set-up is 20 times more productive than similar systems tested previously.
The team also reports that their system can operate without interruption as long as the bacterial cells are replenished each day and the plastic product is removed to keep the reactions going.
That continuous production would be key to making the system work at industrial scales. So far, the researchers have only tested it for 18 days and produced 1.45 grams worth of polyester.
But the researchers say their integrated system is an improvement on previous batch reactors or other set-ups that can only operate one phase of the reaction at a time and require additional separation and purification steps.
Meanwhile, other biochemical engineers aretrying to enhance C. necator‘s natural ability to produce PHB from CO2 with a few genetic tweaks because they say the amount of polymer produced by C. necator is still too low for commercialization – at least for now.
The study has been published in PNAS.