Products You May Like
The steady drum of a heartbeat is something that can easily fade into the background of our daily lives, with little thought given to the reliability of its pulse.
But for those who develop an arrhythmia, the situation is much more fraught – their heartbeat is monitored and controlled by life-saving devices called pacemakers, which deliver controlled shocks to the heart to cease its quivering and give it a chance to find its rhythm again.
Studying the wide variety of ways a heart can stumble and stutter its way into a quivering mess often makes use of small animals as models. But in spite of their convenience and ethical benefits, their size can make it hard to monitor and respond to changes in their tiny hearts.
Now, a team of scientists led by biomedical engineer Philipp Gutruf of the University of Arizona has developed an implantable device that makes studying the cardiology of small animals a lot easier. As a bonus, it could one day be the basis of a whole new way to treat heart conditions in humans.
The device has been designed to be flexible enough for smaller test subjects, providing better resolution for monitoring their heart’s electrophysiology. Using light instead of electrical signals, it administers gentler jolts when abnormal rhythms are detected.
Unlike the electrical signals of current pacemakers, which can interfere with recording capabilities and leave physicians with a patchy picture of cardiac episodes, using light to stimulate the heart means the system can provide continuous recordings of heartbeat patterns – even when it needs to defibrillate.
“Current pacemakers record basically a simple threshold, and they will tell you, ‘This is going into arrhythmia, now shock!'” explains Gutruf.
“But this device has a computer on board where you can input different algorithms that allow you to pace in a more sophisticated way. It’s made for research.”
The device has so far only been tested in mice, but researchers designed it to deliver more precise – and possibly less painful – stimulation of the heart.
It works by using a technique called optogenetics, whereby excitable cells like those in the heart or brain can be activated on demand using light. In this case, mouse cardiomyocytes (heart muscle cells) were genetically engineered to express a membrane-bound protein sensitive to blue light. Switch on the light, and the cells jump into action.
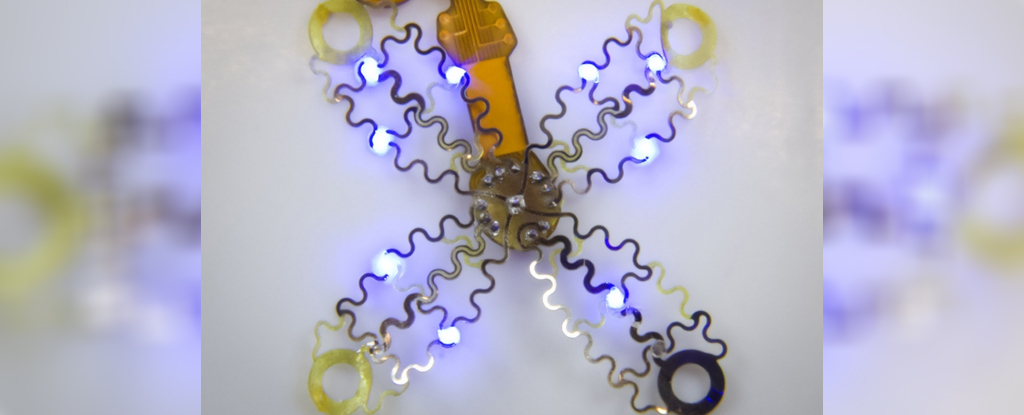
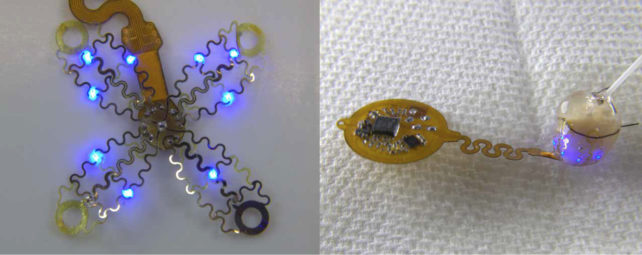
The beauty of the device lies in its soft thin-film arrays, which fan out like flower petals and envelope the heart. This snug fit is rather different to how current pacemakers connect to the heart through one or two leads implanted into the organ.
People who have pacemakers inserted can experience discomfort, and cramping pain around the implantation site. In some rare cases, they may even develop complex regional pain syndrome in the chest.
Stimulating the heart through one or two contact points also makes defibrillation of the heart less precise than is ideal.
“All of the cells inside the heart get hit at one time, including the pain receptors, and that’s what makes pacing or defibrillation painful,” explains Gutruf. “It affects the heart muscle as a whole.”
Instead, with this new device activating only the heart muscle cells that trigger contraction and bypassing pain receptors, the researchers hope it could offer a more comfortable and precise way of synchronizing irregular heartbeats.
“Whereas right now, we have to shock the whole heart to do this, these new devices can do much more precise targeting, making defibrillation both more effective and less painful,” says Igor Efimov, a biomedical engineer at Northwestern University.
As the team describes in their paper, the prototype device was implanted just outside the ribcage of the mouse using a custom-made applicator and a single suture, sharing heart rate data via an infrared uplink.
The researchers first analyzed the geometry and mechanics of a beating mouse heart, using that information to design and laser-fabricate the flexible, four-pronged mesh so it could move with the heart as it thumped out a beat.
Testing the wireless device in freely moving mice, the researchers showed the device could detect abnormal rhythms and stimulate or ‘pace’ the heart with millisecond precision – and without the heat from the light pulses damaging cardiac tissue.
The accuracy of the device in detecting abnormal heartbeats was also comparable to current commercially available wireless heart rate monitoring devices, the researchers report.
Although the findings from animal studies like this one are promising, it’s still very early days for the use of optogenetics in humans. The technique requires gene therapy (to make cells light-sensitive) as well as an implantable electronic device to stimulate them in a controlled way.
While optogenetics has been used in clinical trials to treat rare inherited eye diseases, using the technique to monitor and possibly treat heart irregularities remains an emerging approach that needs a lot more research in animals first.
There are a host of challenges to overcome, including the safe and effective delivery of genetic instructions encoding light-sensitive proteins to heart cells.
More work also needs to be done to model the intricacies of heart rhythm disorders and to refine this particular device’s modalities for sensing and correcting different kinds of arrhythmias, Gutruf and colleagues note.
So, for the moment, the flower-like device is one of many that presents an elegant research tool for studying arrhythmias and other heart problems as they happen, at least in animal models.
The study was published in Science Advances.