Products You May Like
Water gets boiled a lot – whether it’s a cup of tea brewing in a kitchen or a power plant generating electricity. Any improvements in the efficiency of this process will have a huge impact on the overall amount of energy used for it every day.
One such improvement could come with a newly developed treatment for surfaces involved in heating and evaporating water. The treatment improves two key parameters that determine the boiling process: the heat transfer coefficient (HTC) and the critical heat flux (CHF).
Most of the time, there’s a trade-off between the two – as one improves, the other gets worse. After years of investigation, the research term behind the technique has found a way of enhancing both.
“Both parameters are important, but enhancing both parameters together is kind of tricky because they have intrinsic trade-off,” says bioinformatics scientist Youngsup Song from the Lawrence Berkeley National Laboratory in California.
“If we have lots of bubbles on the boiling surface, that means boiling is very efficient, but if we have too many bubbles on the surface, they can coalesce together, which can form a vapor film over the boiling surface.”
Any vapor film between the hot surface and the water introduces resistance, lowering the heat transfer efficiency and the CHF value. To get around the issue, the researchers devised three different kinds of surface modification.
First, a series of microscale tubes are added. This array of 10-micrometer-wide tubes, spaced about 2 millimeters apart, controls bubble formation and keeps the bubbles pinned to the cavities. That prevents a vapor film from forming.
At the same time, it reduces the concentration of bubbles on the surface, reducing boiling efficiency. To tackle that, the researchers introduced an even smaller-scale treatment as the second modification, adding bumps and ridges just nanometers in size within the surface of the hollow tubes. That increases the available surface area and promotes evaporation rates.
Lastly, the microscale cavities were housed in the center of a series of pillars on the material surface. These pillars speed up the drawing-off process for the liquid by adding more surface area. In combination, the boiling efficiency is significantly increased.
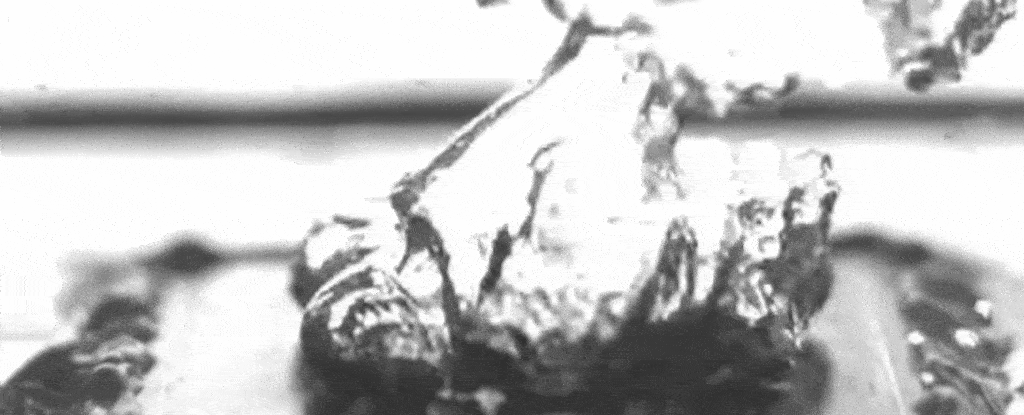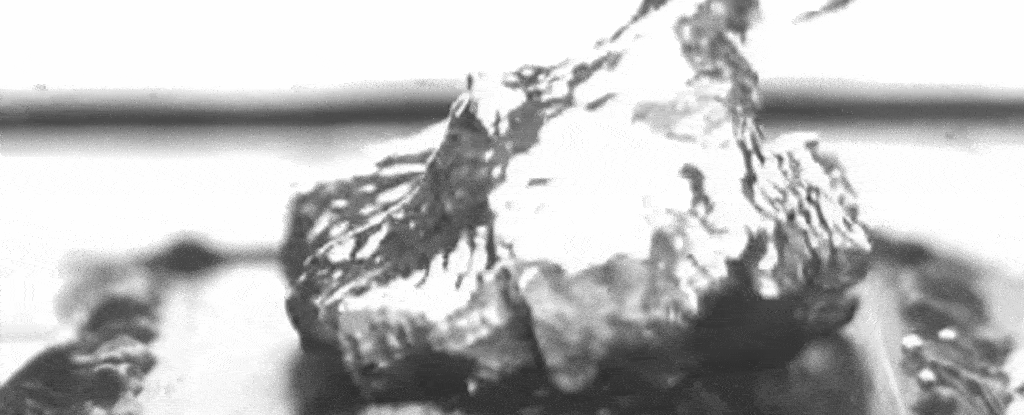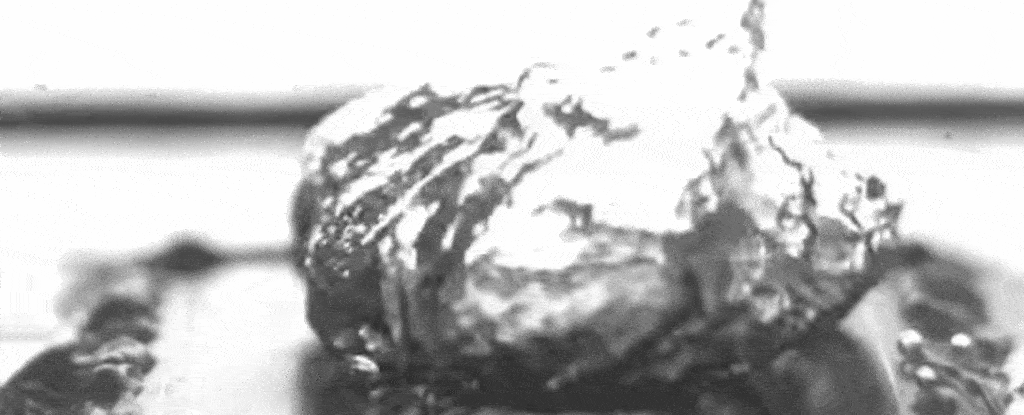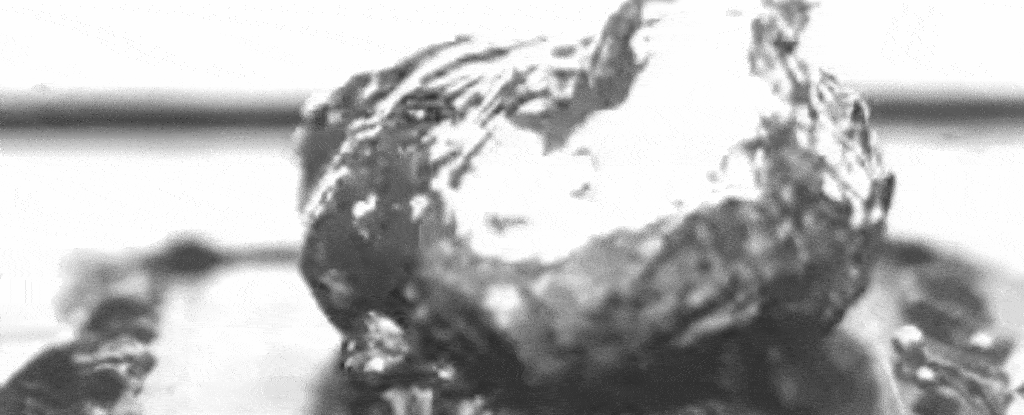
Above: A slowed-down video of the researchers’ set-up shows water boiling on a specially treated surface that causes bubbles to form at specific separate points.
As the nanostructures also promote evaporation under the bubbles, and the pillars keep up a steady supply of liquid to that bubble base, a layer of water between the boiling surface and the bubbles can be maintained – enhancing the maximum heat flux.
“Showing that we can control the surface in this way to get enhancement is a first step,” says mechanical engineer Evelyn Wang from the Massachusetts Institute of Technology. “Then the next step is to think about more scalable approaches.”
“These kinds of structures we’re making are not meant to be scaled in their current form.”
Taking the work from a small-scale laboratory setting into something that can be used in commercial industries won’t be all that straightforward, but the researchers are confident that it can be done.
One challenge is going to be finding ways of creating the surface textures and the three “tiers” of modifications. The good news is that there are different approaches that can be explored, and the procedure should work for different kinds of liquids too.
“Those kinds of details can be changed, and that can be our next step,” says Song.
The research has been published in Advanced Materials.