Products You May Like
When microbiologist Jared Leadbeater returned to his office for the first time in months after a work trip, he found something strange. A cream-coloured manganese carbonate (MnCO3) compound, coating glassware he’d left soaking in his sink, had turned dark. Something had stolen some of its electrons.
“I thought, ‘What is that?'” said Leadbeater, a researcher at the California Institute of Technology (Caltech). The dark substance was a form of manganese oxide - a product that forms when manganese ions lose electrons and undergo a reaction called oxidation.
But something had to be initiating the reaction – an electron thief.
“I started to wonder if long-sought-after microbes might be responsible,” Leadbeater explained, “so we systematically performed tests to figure that out.”
To check if this was really happening due to a biological process, Leadbeater and his team coated more jars with MnCO3 and sterilised some them using scorching steam (MnCO3 is known to be stable in these conditions).
The manganese compound on those didn’t darken (even a year later), but the flasks that hadn’t been sterilised did. Therefore, the electron thief had to be something that could be destroyed by hot steam.
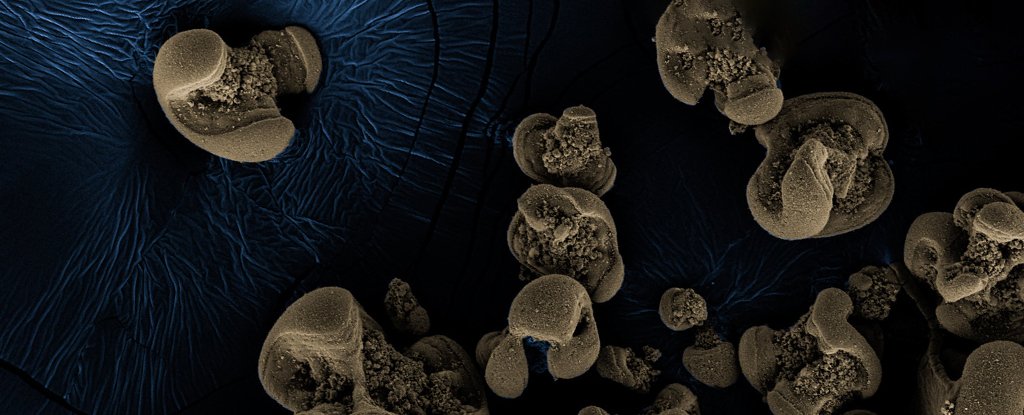
So, the researchers cultured what was on the jars. RNA analysis revealed 70 species of bacteria, but with further tests the team managed to rule some out, until just two possible culprits remained.
They were Nitrospirae bacteria which is usually crescent-shaped, and the rod-shaped betaproteobacterium. Relatives of both these bacteria species are known to live in groundwater.
“We isolated [the betaproteobacterium] from disrupted oxides as single colonies… but this species does not oxidise MnCO3 alone. Either the Nitropirae is solely responsible for Mn(II) oxidation or the activity is consortial,” the team writes in a new study.
The electron theft may have been a team effort, the team realised. But what was the motive? The researchers had their suspicions.
They used manganese labelled with carbon 13 in some of their cultures and, sure enough, the bacteria incorporated these carbon isotopes into their bodies.
This confirmed the suspect bacteria were autotrophic – are able to produce their own food using a source of energy.
The bacteria were using the energy from the manganese electrons to change CO2 into usable carbon, like plants use sunlight to turn CO2 and water into sugars and oxygen during photosynthesis.
This process is called chemosynthesis, and while known to occur using other metals, it’s the first time we’ve seen cells make use of manganese in this way.
“These are the first bacteria found to use manganese as their source of fuel,” explained Leadbetter, although such microbes were predicted to exist over a century ago.
Manganese is an essential nutrient for us as well. Our bodies use it for things like processing fats and proteins and bone formation, and we get it from foods such as nuts and teas and leafy greens.
While it’s one of the most common elements on our planet’s surface, a lot about manganese and its cycle on Earth remains a mystery – including its strange tendency to clog water pipes.
“There is a whole set of environmental engineering literature on drinking-water-distribution systems getting clogged by manganese oxides,” said Leadbetter.
“But how and for what reason such material is generated there has remained an enigma. Clearly, many scientists have considered that bacteria using manganese for energy might be responsible, but evidence supporting this idea was not available until now.”
Manganese oxide also mysteriously appears as nodules across much of the seafloor, and manganese is involved in many interconnected cycles of elements including carbon, nitrogen, iron, and oxygen.
So, the existence of manganese electron-stealing thieves, like these newly discovered bacteria, could explain a lot.
The researchers say the bacteria’s cell doubling times and rates of oxidation would create manganese oxides in amounts equivalent to global reserves in just two years.
Close relatives of these species seem to be present in many places, so their potential to cycle this metal across Earth could be vast.
“This discovery fills a major intellectual gap in our understanding of Earth’s elemental cycles, and adds to the diverse ways in which manganese, an abstruse but common transition metal, has shaped the evolution of life on our planet,” said Caltech geobiologist Woodward Fischer, who was not involved with the study.
This research was published in Nature.